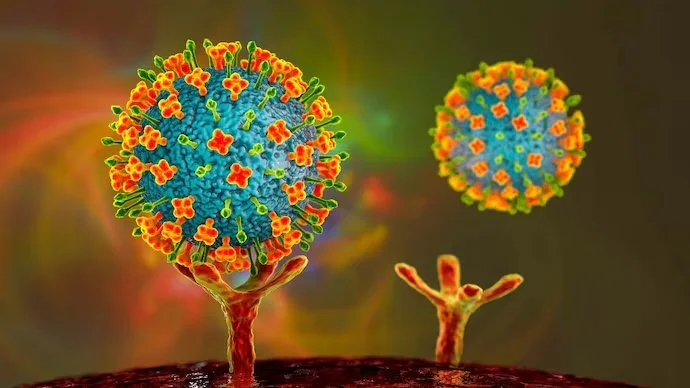
Nipah Virus
Image 1: Nipah virus binding to the host ephrin-B2 receptor.
General Viral Characteristics
The first signs of Nipah virus (NiV) appeared in 1998 amongst Malaysian adult males who farmed pigs (1). The farmers were experiencing encephalitis and respiratory illness (1). Japanese B Encephalitis (JE), a member of the flaviviridae family (4), was suspected of causing the outbreak (1). JE causes similar symptoms to NiV, and JE antibodies appeared in the infected individuals’ serum (1).
However, since JE transmission occurs via mosquitoes that tend to feast on all age groups and genders, it was odd that only adult males were infected (1). Also, JE had a much lower mortality rate than NiV (1). For example, the mortality rate of Nipah was around 40% when authorities stopped its spread in 1998 (1). Now, the mortality rate is at 75% (6). Knowing differences amongst the pathogens, virologists theorized a newly emerging virus may have triggered the outbreak, so studies began at the University of Malaya in 1999 (1).
Researchers extracted cerebral spinal fluid from the deceased patients and concluded that the newly discovered Nipah virus was the culprit. Nipah is part of the Paramyxoviridae family and Henipavirus genus (1). The virus genome contains a coated, nonsegmented, negative-sense RNA. Phosphoproteins attached to viral nucleocapsid proteins make up the viral protein coat (9). The genome contains six genes that code for nine types of proteins (9).
Each vRNP derived from the Nipah genome consists of a nucleocapsid (N), phosphoprotein (P), long polymerase (L), and viral genome. The vRNP is contained within the helical lipid envelope between each noncoding region. The envelope includes the matrix proteins (M) that fasten the fusion glycoproteins (F) and attachment glycoproteins (G) to the envelope (9).
NiV is a zoonotic virus where fruit bats serve as the virus’s primary reservoir (9). Observations of NiV transmission show that consumption of fruit bat excretions directly caused infection (3)(9). For example, it is part of their culture in Malaysia to drink date palms right from the tree. Fruit bats around that area help fertilize the dates and other fruit trees around the farm leading to their secretions falling onto the fruits consumed before being washed (9).
Thus, Nipah is transmitted through the fecal-oral route, like the norovirus, to infect humans and pigs (3). Research shows other mammals may be infected, too. Pigs amplify the virus, which has led to endemics in the past (3)(9). Once the infection has taken route within humans, horizontal transfer is possible, which may be caused by respiratory droplets and bodily excretions.
The Infectious Cycle
The infectious cycle starts when a host has consumed food contaminated by Nipah. Our bodies will digest the foodstuffs and distribute them to various areas around the body via the bloodstream. The bloodstream is where NiV starts its attack. NiV infects many body cells using its viral G proteins for attachment to the host ephrin-B2 or B3 receptor (9). The only difference between the two host receptors is their affinity for the viral G protein (9). Covalent interaction between the host and viral receptors activates the G protein, inducing a conformational change that sanctions the extension of the F protein. This extension permits membrane fusion where the viral envelope is lost, and the vRNP complex is released into the cytoplasm (9).
Here the vRNP serves two enzymatic activities: transcriptase of the viral mRNAs and replicase of the viral genome (6). vRNP transcription begins within the cytosol using its long polymerase (8). The long polymerase serves to cap, methylate, and polyadenylate the viral mRNAs making them resemble host mRNAs so that they can be translated by host enzymes (8). Each vRNP is associated with the genome at the six specific protein-coding regions. Therefore, each vRNP complex gives rise to one of the six viral transcripts that create the major viral proteins of Nipah.
The viral G proteins are synthesized on the rough endoplasmic reticulum (RER) and mature through the Golgi network by glycosyltransferases (8). After maturation, the viral G proteins accumulate on the host’s plasma membrane and await further action. Translation of F proteins also takes place on the RER, but these proteins mature within the host endosome that transfers them to the plasma membrane (8). Translation of the other viral proteins happens on free ribosomes within the host cell.
The viral P gene encodes three viral proteins in addition to the phosphoproteins (8). Post- transcriptional modifications to the P viral transcripts create the viral proteins V and W. A downstream initiation site within the P gene produces NiV protein C (8). These viral proteins may induce repression of minigenome transcription and replication (8). The other viral proteins synthesized on free ribosomes include the N, M, and L proteins.
N proteins accumulate in the cytosol, forming new vRNP complexes with NiV L and P proteins. Lastly, after creating viral M protein, they migrate to the host nucleus, then the plasma membrane (7). Why M proteins travel to the nucleus is not understood, but it is necessary for viral replication (7). If mutations occur within the genes that regulate M proteins transfer to and from the nucleus, viral replication cannot progress to the lytic state (7). Another thing not fully understood is what activates vRNP replicase activity.
Although, once vRNP replicase is activated, it generates a full-length, single-stranded, positive- sense, anti-genomic RNA strand from the primary viral genome (8). The vRNP will link to the anti- genomic strand and construct nascent viral genomes. Niv N proteins then bring the genomes to the viral envelope proteins at the cell surface (8). When the genome contacts the viral matrix proteins at the cell’s surface, it may activate them. Theories suggest that matrix proteins interact with the cytoplasmic tails of the F and G viral proteins, releasing the viral progenies into the extracellular space (8).
Characteristics of Illness and the Disease
Nipah virus infections range from moderate to severe (10). When symptoms first present, they are flulike, consisting of headache, sore throat, fever, lethargy, and vomiting (10). Shortly after, encephalitis begins, and patients lose coordination, neurologic control, and consciousness (10). Also, some patients experience acute respiratory distress during this time (10).
NiV infects almost all body cells due to the ephrin receptor on most of them (2)(11). This viral tropism may lead to a systemic infection. The incubation period of Nipah is not exact but is usually between 4-14 days (10). When contaminated food is eaten, epithelial cells of the digestive tract are most likely the first to be hit by NiV. After the virus replicates, it can enter the bloodstream and infect even more cells (10).
Then, the virus continues to spread, eventually invading endothelial cells. When blood vessels lyse from viral replication, it destroys the blood-brain barrier (10). Now, the virus has access to the central nervous system, leading to the inflammatory response in the brain. The virus can get this far in infection due to its viral V, W, and C proteins, which also deactivate the host cell’s immune responses (8). They act like mRNA sponges that bind to host genes that transduce immune reactions (10).
Although, once the virus gets farther into this replication cycle, those proteins are needed for other activities. So, the immune response is no longer suppressed, but it is still too late. As a result, a cytokine storm attacks the body and its organs (10). The late cytokine storm gives rise to the high mortality rate among patients.
Treatment and Prevention for Nipah Virus
There are no approved vaccines, but researchers are finding promising results. They have manufactured an “epitope-based peptide vaccine” (4). The peptide, FLIDRINWI, shows a strong binding affinity to MHC I and MHC II receptors on immune cells (4). The binding could inhibit the effects of the cytokine storm, ultimately neutralizing the immune response.
However, since the vaccine is not yet approved, prevention measures are still considered. These include washing hands after touching livestock and routine cleaning on farms (9). Other preventive measures include isolating infected individuals, washing produce before consumption, culling infected animals, and other necessary aseptic techniques (9).
References
(1) Ang, B., Lim, T., & Wang, L. (2018). Nipah Virus Infection. Journal of clinical microbiology, 56(6), e01875-17. https://doi.org/10.1128/JCM.01875-17
(2) biotechne. (2022). Ephrin-B2: A receptor for Henipaviruses. www.rndsystems.com. Retrieved March 23, 2022, from https://www.rndsystems.com/resources/articles/ephrin-b2-receptor- henipaviruses
(3) Epstein, J. H., Anthony, S. J., Islam, A., Kilpatrick, A. M., Ali Khan, S., Balkey, M. D., Ross, N., Smith, I., Zambrana-Torrelio, C., Tao, Y., Islam, A., Quan, P. L., Olival, K. J., Khan, M., Gurley, E. S., Hossein, M. J., Field, H. E., Fielder, M. D., Briese, T., Rahman, M., ... Daszak, P. (2020). Nipah virus dynamics in bats and implications for spillover to humans. Proceedings of the National Academy of Sciences of the United States of America, 117(46), 29190–29201. https://doi.org/10.1073/pnas.2000429117
(4) Kraft, S. (2018, August 28). Japanese encephalitis: Symptoms, treatment, transmission, and more. Medical News Today. Retrieved March 22, 2022, from https://www.medicalnewstoday.com/articles/181418
(5) Mohammed, A. A., Shantier, S. W., Mustafa, M. I., Osman, H. K., Elmansi, H. E., Osman, I. A., Mohammed, R. A., Abdelrhman, F. A., Elnnewery, M. E., Yousif, E. M., Mustafa, M. M., Elfadol, N. M., Abdalla, A. I., Mahmoud, E., Yagaub, A. A., Ahmed, Y. A., & Hassan, M. A. (2020). Epitope- Based Peptide Vaccine against Glycoprotein G of Nipah Henipavirus Using Immunoinformatics Approaches. Journal of immunology research, 2020, 2567957. https://doi.org/10.1155/2020/2567957
(6) Ranadheera, C., Proulx, R., Chaiyakul, M., Jones, S., Grolla, A., Leung, A., Rutherford, J., Kobasa, D., Carpenter, M., & Czub, M. (2018). The interaction between the Nipah virus nucleocapsid protein and phosphoprotein regulates virus replication. Scientific reports, 8(1), 15994. https://doi.org/10.1038/s41598-018-34484-7
(7) Ringel M, Behner L, Heiner A, Sauerhering L, Maisner A. Replication of a Nipah Virus Encoding a Nuclear-Retained Matrix Protein. J Infect Dis. 2020 May 11;221(Suppl 4):S389-S394. doi: 10.1093/infdis/jiz440. PMID: 31665345.
(8) Rota PA, Lo MK. Molecular virology of the henipaviruses. Curr Top Microbiol Immunol. 2012;359:41-58. doi: 10.1007/82_2012_211. PMID: 22552699.
(9) Singh, R. K., Dhama, K., Chakraborty, S., Tiwari, R., Natesan, S., Khandia, R., Munjal, A., Vora, K. S., Latheef, S. K., Karthik, K., Singh Malik, Y., Singh, R., Chaicumpa, W., & Mourya, D. T. (2019). Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies - a comprehensive review. The veterinary quarterly, 39(1), 26– 55. https://doi.org/10.1080/01652176.2019.1580827
(10)Upendrababu, V. (2018). Nipah Virus Infection, a High Priority Disease: History, Facts, Transmission, Symptoms, Prevention and Treatment.
(11)U.S. National Library of Medicine. (2022, March 13). EFNB2 ephrin B2 [Homo Sapiens (human)] - gene - NCBI. National Center for Biotechnology Information. Retrieved March 23, 2022, from https://www.ncbi.nlm.nih.gov/gene/1948
The Resurrection of Viruses
Image 1: A virus, red blood cells, and a double helix.
Breakthroughs and Complications
Issue Summary and Analysis
Climate change is something everyone is familiar with, but what does this phenomenon hold for the future of humanity? At this point, global warming is not just about how the world is overheating. There are even greater potential threats (Houwenhuyse et al., 2017). Yes, the ice caps are melting and releasing sequestered carbon into the atmosphere. However, carbon is not the only thing permafrost liberates. There are also tiny particles, called viruses, that reside within the ice (Doucleff, 2016).
Viruses are more abundant in number than any other species on Earth. These particles even live inside humans and animals, like bacteria and the microbiome (Houwenhuyse et al., 2017). Therefore, since they coexist within and among humanity, not all viruses are necessarily harmful (Houwenhuyse et al., 2017). Some of these pathogens are even useful in treating certain cancers (Houwenhuyse et al., 2017). However, viruses used for such treatments have been genetically modified to no longer carry infectious genes. Yet, the particles arising on their own from the mid to high altitude regions of Earth will still hold their virulent genes and, theoretically, their pathogenicity (Doucleff, 2016).
Knowing this, researchers from all over the globe decided to resurrect these infectious particles for analysis (Becker, 2017). They did this by taking ice core samples from the arctic back to the lab. Then, they would place specific microorganisms within the ice core to determine if there were pathogenic particles within. For example, Pithovirus sibericum, a 300,000-year-old disease, was resurrected from the permafrost and still showed its infective nature (Houwenhuyse et al., 2017). Researchers discovered this by first placing an amoeba on the ice core sample and then observing the organism under the microscope, where it was infected with the prehistoric virus (Houwenhuyse et al., 2017).
Thus, researchers, including Dr. Tumpey, a research scientist at the Centers for Disease Control and Prevention in Atlanta, believe it is necessary experimentation that could lead to a greater understanding of viruses and their evolution (Shreeve, 2006). However, with the possibility of these pathogens harming the public if they escape, bioethicists have raised a need for a “greater international review” before more research continues (Shreeve, 2006).
Richard H. Ebright, a molecular biologist, agrees with the bioethicists’ decision due to the potential use of these pathogens as “bioweapons” (Kolata, 2005). Although officials assured the public that scientists with Biosafety Level 4 clearance were performing the research in highly secured laboratories, the verdict remains as to whether the investigation and breakthroughs that come with it outweigh the harm these pathogens could do if placed in the wrong hands (Kolata, 2005; Shreeve, 2006).
Annotated Bibliography
Becker, R. (2017, July 7). Resurrecting an extinct relative of smallpox could pave the way for better vaccines. The Verge. Retrieved March 19, 2022, from https://www.theverge.com/2017/7/7/15938168/smallpox-variola-horsepox-virus-synthesis-vaccines-bioweapon
David Evans, a microbiologist, has resurrected the horsepox virus. This virus is a relative of the once deadly smallpox. If specific genetic modifications were made to this virus, it has the potential for use as a bioweapon by terrorists. When he published his paper, there was controversy about whether his findings were safe to share. But, ultimately, other professionals in the field agreed his work should be published because it could potentially help many people suffering from genetic disorders and certain cancers.
Doucleff, M. (2016, August 3). Anthrax outbreak in Russia thought to be result of thawing permafrost. npr. Retrieved March 24, 2022, from https://www.npr.org/sections/goatsandsoda/2016/08/03/488400947/anthrax-outbreak-in-russia-thought-to-be-result-of-thawing-permafrost
More things are resurfacing from the permafrost as the climate changes, including viruses. For example, recent anthrax outbreaks have occurred in Russia due to high heat melting the ice during the summer. For centuries bodies of deceased, infected individuals have been buried under this thick layer of ice. Researchers now believe that many other pathogens, even those prehistoric, could cause infectious diseases to spread across the world due to the progressively changing climate.
Houwenhuyse, S., Macke, E., Reyserhove, L., Bulteel, L., & Decaestecker, E. (2017). Back to the future in a petri dish: Origin and impact of resurrected microbes in natural populations. Evolutionary Applications, 2018 (11), 29-41. Retrieved February 20, 2022, from DOI: 10.1111/eva.12538
Climate change has caused the permafrost to melt in mid to high-altitude regions, leading to the reemergence of historically relevant and ancient pathogens into society. Work done in labs to resurrect these viruses led to many discoveries. Some being that microorganisms have evolved “multi-drug resistant genes” over centuries. Also, studying the evolution of current viruses is not something that comes easy because it does not happen fast. Therefore, having the ability to resurrect the ones from prehistoric times will lead to more significant strides in evolutionary virology.
Kolata, G. (2005, October 6). Experts unlock clues to spread of 1918 flu virus. The New York Times. Retrieved March 24, 2022, from https://www.nytimes.com/2005/10/06/health/experts-unlock-clues-to-spread-of-1918-flu-virus.html?searchResultPosition=2.
The flu that caused the 1918 influenza epidemic was one of the deadliest strains to surface, with a 100% mortality rate. Scientists revived the strain to discover it had gone through recombination with the bird flu, now allowing it to infect humans. Therefore, former President Bush acquired a team of the best virologists in America to study the strain for potential vaccines. Still, civilians and some scientists think this act is of concern due to their possible use as bioweapons. However, virologists and Bush reassure that this is a way to produce treatment options “before it is too late.”
Shreeve, J. (2006, January 29). Why revive a deadly flu virus? The New York Times. Retrieved March 13, 2022, from https://www.nytimes.com/2006/01/29/magazine/why-revive-a-deadly-flu-virus.html?searchResultPosition=4.
Dr. Tumpey, a research scientist, works with “highly pathogenic microbes,” including the resurrected 1918 flu, and he faces controversy every day for it. However, almost all scientists believe this work will pay off. Understanding the reasons that make this virus and others so pathogenic allows vaccines and treatment options to be discovered before the population is in danger. It will happen whether the work is done in the lab or by nature itself. Tumpey understands the potential risks but believes that this work is necessary for the health of humanity.